
Table 6 from Pd(0)-guanidine@MCM-41 as efficient and reusable heterogeneous catalyst for C–C coupling reactions | Semantic Scholar
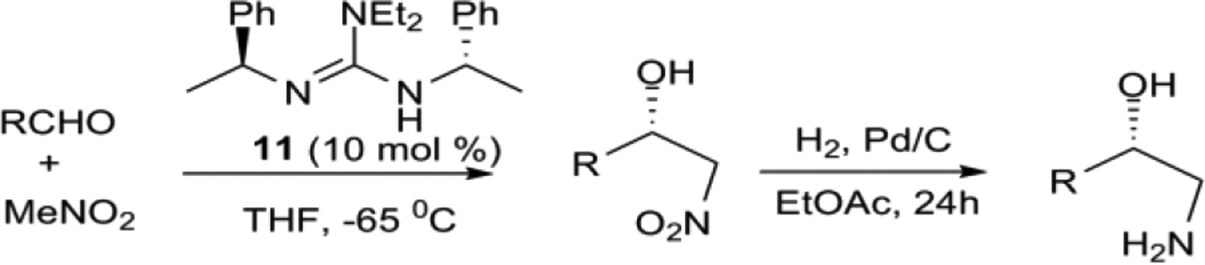
Recent Advances in Guanidine-Based Organocatalysts in Stereoselective Organic Transformation Reactions | IntechOpen

Pd(0)‐guanidine@MCM‐41: a very effective catalyst for rapid production of bis (pyrazolyl)methanes - Filian - 2020 - Applied Organometallic Chemistry - Wiley Online Library
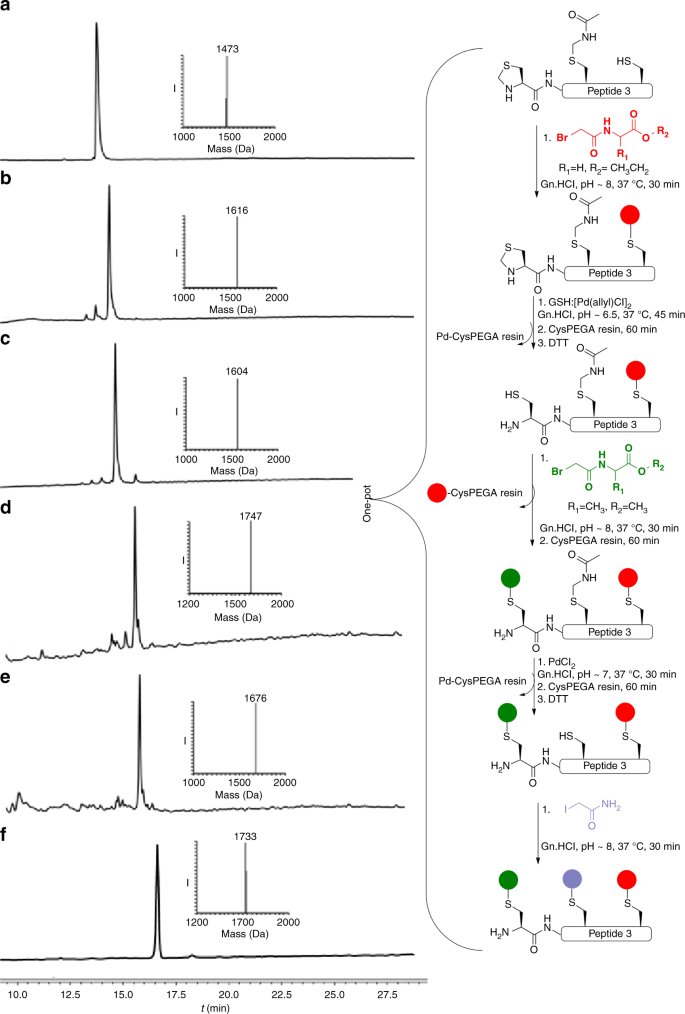
Palladium prompted on-demand cysteine chemistry for the synthesis of challenging and uniquely modified proteins | Nature Communications

Asymmetric NH Insertion of Secondary and Primary Anilines under the Catalysis of Palladium and Chiral Guanidine Derivatives - Zhu - 2014 - Angewandte Chemie International Edition - Wiley Online Library
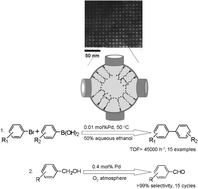
Palladium-guanidine complex immobilized on SBA-16: a highly active and recyclable catalyst for Suzuki coupling and alcohol oxidation - Green Chemistry (RSC Publishing)
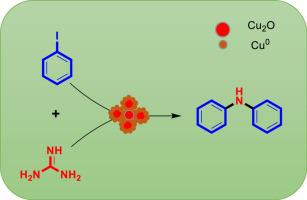
One-pot synthesis of symmetrical and asymmetrical diphenylamines from guanidines with aryl iodide using Cu/Cu2O nanocatalyst - Mol. Catal. - X-MOL

Pd(0)‐guanidine@MCM‐41: a very effective catalyst for rapid production of bis (pyrazolyl)methanes - Filian - 2020 - Applied Organometallic Chemistry - Wiley Online Library
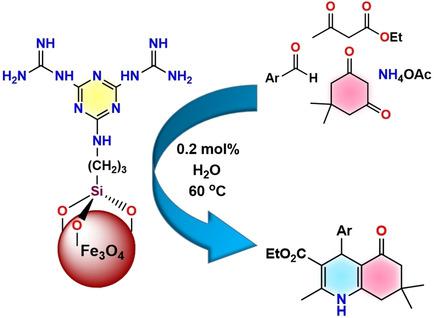
Simple and Efficient Synthesis of Guanidine‐Based Magnetic Nanocatalyst for the One‐Pot, Four‐Component Synthesis of Polyhydroquinolines in Water,ChemistrySelect - X-MOL
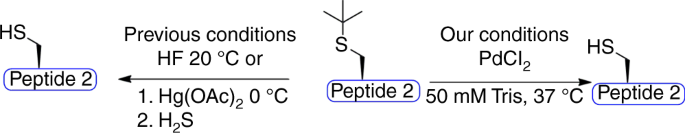
Palladium prompted on-demand cysteine chemistry for the synthesis of challenging and uniquely modified proteins | Nature Communications

Table 5 from Pd(0)-guanidine@MCM-41 as efficient and reusable heterogeneous catalyst for C–C coupling reactions | Semantic Scholar

Pd(0)‐guanidine@MCM‐41: a very effective catalyst for rapid production of bis (pyrazolyl)methanes - Filian - 2020 - Applied Organometallic Chemistry - Wiley Online Library
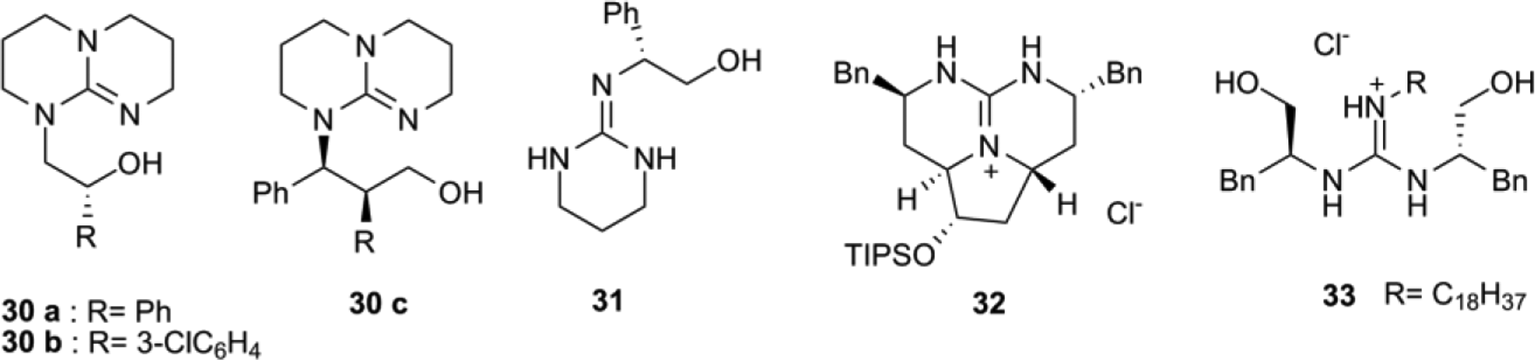
Recent Advances in Guanidine-Based Organocatalysts in Stereoselective Organic Transformation Reactions | IntechOpen

Brønsted Guanidine Acid-Base Ionic Liquids: Novel Reaction Media for the Palladium-Catalyzed Heck Reaction

Guanidine/Pd(OAc)2-Catalyzed Room Temperature Suzuki Cross-Coupling Reaction in Aqueous Media under Aerobic Conditions
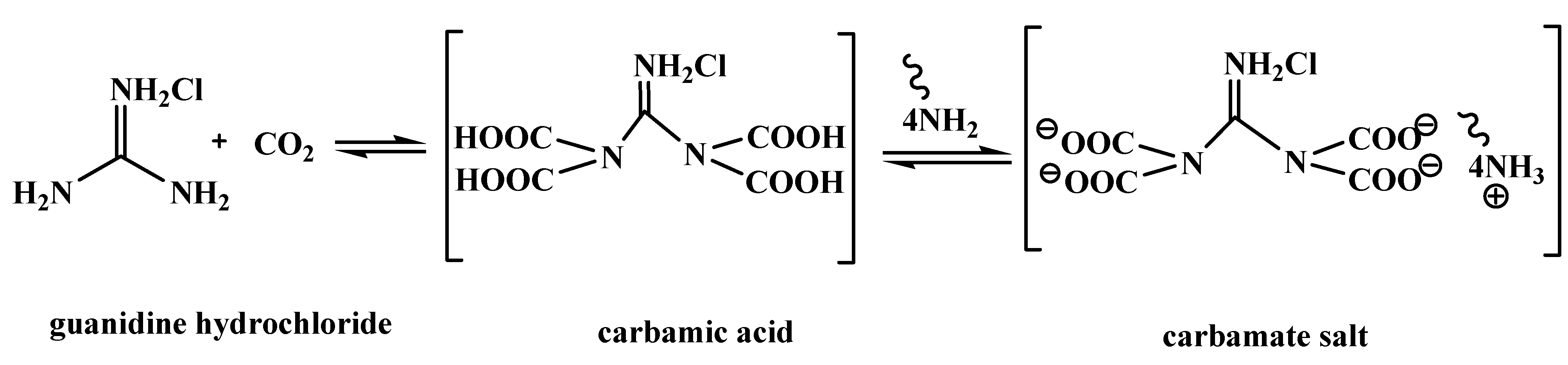
Catalysts | Free Full-Text | Guanidine Hydrochloride/ZnI2 as Heterogeneous Catalyst for Conversion of CO2 and Epoxides to Cyclic Carbonates under Mild Conditions

Pd(0)‐guanidine@MCM‐41: a very effective catalyst for rapid production of bis (pyrazolyl)methanes - Filian - 2020 - Applied Organometallic Chemistry - Wiley Online Library

Table 2 from Self-liganded Suzuki-Miyaura coupling for site-selective protein PEGylation. | Semantic Scholar



